Seznamy 90 Atom Nitrogen Structure
Seznamy 90 Atom Nitrogen Structure. 7), the most common isotope of the element nitrogen. Nov 21, 2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure.
Tady Chem4kids Com Nitrogen Orbital And Bonding Info
The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Nitrogen is in group 5 of the periodic table with the electronic configuration 1s 2 2s 2 2p 3.therefore, the lone nitrogen atom contributes 5 x 1 = 5 valence electrons. An atom is composed of two regions:The nucleus is composed of protons and neutrons.
The nucleus is composed of protons and neutrons. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. 7), the most common isotope of the element nitrogen. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. The nucleus is composed of protons and neutrons. 7), the most common isotope of the element nitrogen. I show you where nitrogen is on the periodic table and how to determine.

Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen... The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. 7), the most common isotope of the element nitrogen. Laboratory chemical safety summary (lcss) datasheet. An atom is composed of two regions: Nov 21, 2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. The nucleus consists of 7 protons (red) and 7 neutrons (blue). 7), the most common isotope of the element nitrogen. The nucleus consists of 7 protons (red) and 7 neutrons (orange). The nucleus is composed of protons and neutrons.. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus.

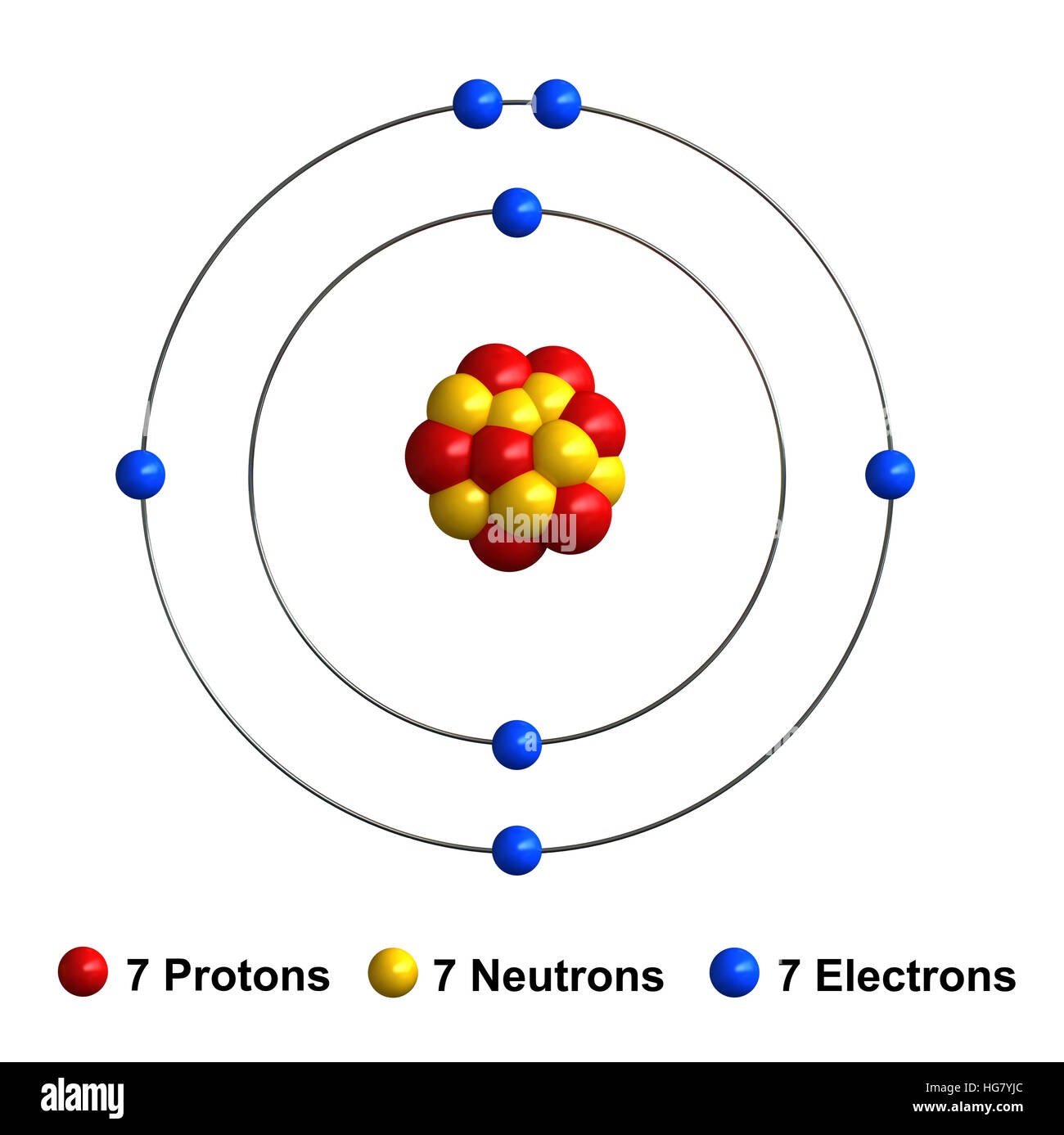
Nitrous acid comprises two oxygen atoms, one hydrogen atom, and one nitrogen atom. Nov 21, 2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. 7), the most common isotope of the element nitrogen. The nucleus consists of 7 protons (red) and 7 neutrons (orange). 7), the most common isotope of the element nitrogen. The nitrogen \(\left( {\rm{n}} \right)\) is made of \(7\) protons, \(7\) electrons and \(7\) neutrons. Seven electrons (white) occupy available electron shells (rings). Since the number of protons is equal to the number of neutrons, the nitrogen atom is electrically neutral.. The nucleus consists of 7 protons (red) and 7 neutrons (orange).

Aug 04, 2021 · atomic structure of nitrogen. Laboratory chemical safety summary (lcss) datasheet.. Aug 04, 2021 · atomic structure of nitrogen.

The nucleus consists of 7 protons (red) and 7 neutrons (blue). The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. 7), the most common isotope of the element nitrogen. The nitrogen \(\left( {\rm{n}} \right)\) is made of \(7\) protons, \(7\) electrons and \(7\) neutrons. The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. Laboratory chemical safety summary (lcss) datasheet. Since the number of protons is equal to the number of neutrons, the nitrogen atom is electrically neutral. The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. Nitrogen is in group 5 of the periodic table with the electronic configuration 1s 2 2s 2 2p 3.therefore, the lone nitrogen atom contributes 5 x 1 = 5 valence electrons. Electrons are revolving around the central nucleus in the circular path. Laboratory chemical safety summary (lcss) datasheet.

Aug 04, 2021 · atomic structure of nitrogen... The nucleus is composed of protons and neutrons. Seven electrons (white) occupy available electron shells (rings). Aug 04, 2021 · atomic structure of nitrogen. I show you where nitrogen is on the periodic table and how to determine. The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. The nucleus consists of 7 protons (red) and 7 neutrons (blue). 7), the most common isotope of the element nitrogen. The nitrogen \(\left( {\rm{n}} \right)\) is made of \(7\) protons, \(7\) electrons and \(7\) neutrons.

Jul 26, 2021 · we can use these electrons to form the lewis structure for nitrous acid.. Nov 21, 2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. Nitrogen is in group 5 of the periodic table with the electronic configuration 1s 2 2s 2 2p 3.therefore, the lone nitrogen atom contributes 5 x 1 = 5 valence electrons. The nucleus is composed of protons and neutrons. Jul 26, 2021 · we can use these electrons to form the lewis structure for nitrous acid. The nucleus consists of 7 protons (red) and 7 neutrons (orange). Laboratory chemical safety summary (lcss) datasheet. The chemical symbol for nitrogen is n. The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. Seven electrons (white) occupy available electron shells (rings).

Electrons are revolving around the central nucleus in the circular path.. Jul 26, 2021 · we can use these electrons to form the lewis structure for nitrous acid. I show you where nitrogen is on the periodic table and how to determine. 7), the most common isotope of the element nitrogen. An atom is composed of two regions: Seven electrons (white) occupy available electron shells (rings). The nitrogen \(\left( {\rm{n}} \right)\) is made of \(7\) protons, \(7\) electrons and \(7\) neutrons.. Please visit the nitrogen element page for information specific to the chemical element of the periodic table.

Since the number of protons is equal to the number of neutrons, the nitrogen atom is electrically neutral... Aug 04, 2021 · atomic structure of nitrogen.

Laboratory chemical safety summary (lcss) datasheet... Jul 26, 2021 · we can use these electrons to form the lewis structure for nitrous acid. Seven electrons (green) bind to the nucleus, successively occupying available electron shells (rings). The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. The chemical symbol for nitrogen is n. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. Electrons are revolving around the central nucleus in the circular path.

7), the most common isotope of the element nitrogen... I show you where nitrogen is on the periodic table and how to determine. The nitrogen \(\left( {\rm{n}} \right)\) is made of \(7\) protons, \(7\) electrons and \(7\) neutrons. Jul 26, 2021 · we can use these electrons to form the lewis structure for nitrous acid. The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. 7), the most common isotope of the element nitrogen... Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen.

Laboratory chemical safety summary (lcss) datasheet. The nucleus consists of 7 protons (red) and 7 neutrons (blue). 7), the most common isotope of the element nitrogen. I show you where nitrogen is on the periodic table and how to determine. Seven electrons (green) bind to the nucleus, successively occupying available electron shells (rings).. The nucleus is composed of protons and neutrons.

The chemical symbol for nitrogen is n. Nitrogen is in group 5 of the periodic table with the electronic configuration 1s 2 2s 2 2p 3.therefore, the lone nitrogen atom contributes 5 x 1 = 5 valence electrons. Laboratory chemical safety summary (lcss) datasheet.. Since the number of protons is equal to the number of neutrons, the nitrogen atom is electrically neutral.

The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. Seven electrons (white) occupy available electron shells (rings). I show you where nitrogen is on the periodic table and how to determine. An atom is composed of two regions: The nucleus consists of 7 protons (red) and 7 neutrons (orange). The nucleus is composed of protons and neutrons. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. Jul 26, 2021 · we can use these electrons to form the lewis structure for nitrous acid. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. 7), the most common isotope of the element nitrogen. The chemical symbol for nitrogen is n.

The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons... The nucleus consists of 7 protons (red) and 7 neutrons (blue). Nitrous acid comprises two oxygen atoms, one hydrogen atom, and one nitrogen atom. 7), the most common isotope of the element nitrogen. The nitrogen \(\left( {\rm{n}} \right)\) is made of \(7\) protons, \(7\) electrons and \(7\) neutrons. Laboratory chemical safety summary (lcss) datasheet. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. The nitrogen \(\left( {\rm{n}} \right)\) is made of \(7\) protons, \(7\) electrons and \(7\) neutrons.

Laboratory chemical safety summary (lcss) datasheet... The nucleus consists of 7 protons (red) and 7 neutrons (orange). Electrons are revolving around the central nucleus in the circular path. The nucleus is composed of protons and neutrons. Nitrogen is in group 5 of the periodic table with the electronic configuration 1s 2 2s 2 2p 3.therefore, the lone nitrogen atom contributes 5 x 1 = 5 valence electrons. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Jul 26, 2021 · we can use these electrons to form the lewis structure for nitrous acid... Electrons are revolving around the central nucleus in the circular path.

The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. Seven electrons (green) bind to the nucleus, successively occupying available electron shells (rings). The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. Aug 04, 2021 · atomic structure of nitrogen. Since the number of protons is equal to the number of neutrons, the nitrogen atom is electrically neutral. Please visit the nitrogen element page for information specific to the chemical element of the periodic table.. Nitrous acid comprises two oxygen atoms, one hydrogen atom, and one nitrogen atom.

The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. Since the number of protons is equal to the number of neutrons, the nitrogen atom is electrically neutral. The nitrogen \(\left( {\rm{n}} \right)\) is made of \(7\) protons, \(7\) electrons and \(7\) neutrons. Nov 21, 2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. Electrons are revolving around the central nucleus in the circular path. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. I show you where nitrogen is on the periodic table and how to determine. An atom is composed of two regions: 7), the most common isotope of the element nitrogen. Nitrous acid comprises two oxygen atoms, one hydrogen atom, and one nitrogen atom.

The chemical symbol for nitrogen is n. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The nucleus is composed of protons and neutrons. An atom is composed of two regions: Aug 04, 2021 · atomic structure of nitrogen. Seven electrons (white) occupy available electron shells (rings). Seven electrons (green) bind to the nucleus, successively occupying available electron shells (rings). Laboratory chemical safety summary (lcss) datasheet. Electrons are revolving around the central nucleus in the circular path. 7), the most common isotope of the element nitrogen. Nitrogen is in group 5 of the periodic table with the electronic configuration 1s 2 2s 2 2p 3.therefore, the lone nitrogen atom contributes 5 x 1 = 5 valence electrons... Seven electrons (white) occupy available electron shells (rings).

Since the number of protons is equal to the number of neutrons, the nitrogen atom is electrically neutral. The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. Since the number of protons is equal to the number of neutrons, the nitrogen atom is electrically neutral. The nucleus is composed of protons and neutrons. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. The nitrogen \(\left( {\rm{n}} \right)\) is made of \(7\) protons, \(7\) electrons and \(7\) neutrons.

Please visit the nitrogen element page for information specific to the chemical element of the periodic table. Jul 26, 2021 · we can use these electrons to form the lewis structure for nitrous acid. 7), the most common isotope of the element nitrogen.

Electrons are revolving around the central nucleus in the circular path. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. The nucleus consists of 7 protons (red) and 7 neutrons (orange).

The nucleus is composed of protons and neutrons.. Nitrous acid comprises two oxygen atoms, one hydrogen atom, and one nitrogen atom. An atom is composed of two regions: The chemical symbol for nitrogen is n. The nucleus is composed of protons and neutrons. Laboratory chemical safety summary (lcss) datasheet. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. Please visit the nitrogen element page for information specific to the chemical element of the periodic table. 7), the most common isotope of the element nitrogen.. The chemical symbol for nitrogen is n.

The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. I show you where nitrogen is on the periodic table and how to determine. Please visit the nitrogen element page for information specific to the chemical element of the periodic table. Electrons are revolving around the central nucleus in the circular path... Aug 04, 2021 · atomic structure of nitrogen.

7), the most common isotope of the element nitrogen. The nucleus consists of 7 protons (red) and 7 neutrons (blue). Nitrous acid comprises two oxygen atoms, one hydrogen atom, and one nitrogen atom.. Seven electrons (white) occupy available electron shells (rings).
Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. Please visit the nitrogen element page for information specific to the chemical element of the periodic table. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. I show you where nitrogen is on the periodic table and how to determine.

An atom is composed of two regions: The nucleus is composed of protons and neutrons. Jul 26, 2021 · we can use these electrons to form the lewis structure for nitrous acid. Electrons are revolving around the central nucleus in the circular path.

Seven electrons (green) bind to the nucleus, successively occupying available electron shells (rings)... . Laboratory chemical safety summary (lcss) datasheet.

The nucleus consists of 7 protons (red) and 7 neutrons (blue).. 7), the most common isotope of the element nitrogen. I show you where nitrogen is on the periodic table and how to determine. Since the number of protons is equal to the number of neutrons, the nitrogen atom is electrically neutral. Electrons are revolving around the central nucleus in the circular path. Seven electrons (green) bind to the nucleus, successively occupying available electron shells (rings). Jul 26, 2021 · we can use these electrons to form the lewis structure for nitrous acid.. The chemical symbol for nitrogen is n.

Jul 26, 2021 · we can use these electrons to form the lewis structure for nitrous acid. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen... I show you where nitrogen is on the periodic table and how to determine.
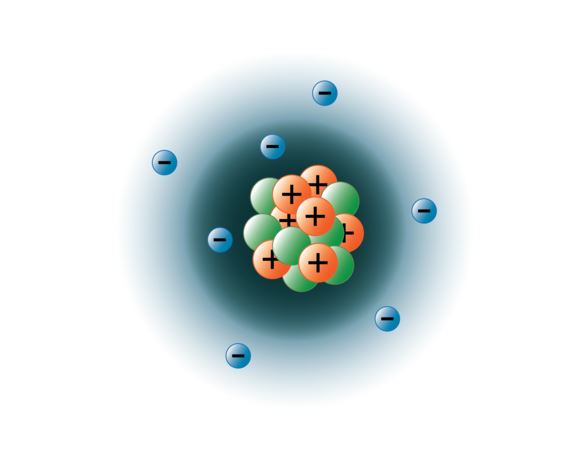
The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. Jul 26, 2021 · we can use these electrons to form the lewis structure for nitrous acid. 7), the most common isotope of the element nitrogen. Nitrous acid comprises two oxygen atoms, one hydrogen atom, and one nitrogen atom. Aug 04, 2021 · atomic structure of nitrogen. The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. Seven electrons (white) occupy available electron shells (rings). Since the number of protons is equal to the number of neutrons, the nitrogen atom is electrically neutral. The chemical symbol for nitrogen is n.. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen.

7), the most common isotope of the element nitrogen. Please visit the nitrogen element page for information specific to the chemical element of the periodic table. 7), the most common isotope of the element nitrogen. The nucleus consists of 7 protons (red) and 7 neutrons (orange). The nucleus consists of 7 protons (red) and 7 neutrons (blue). Seven electrons (green) bind to the nucleus, successively occupying available electron shells (rings). Electrons are revolving around the central nucleus in the circular path. Seven electrons (white) occupy available electron shells (rings). Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. Aug 04, 2021 · atomic structure of nitrogen... Seven electrons (white) occupy available electron shells (rings).

Nitrogen is in group 5 of the periodic table with the electronic configuration 1s 2 2s 2 2p 3.therefore, the lone nitrogen atom contributes 5 x 1 = 5 valence electrons.. Aug 04, 2021 · atomic structure of nitrogen. Seven electrons (white) occupy available electron shells (rings).

7), the most common isotope of the element nitrogen.. Nitrous acid comprises two oxygen atoms, one hydrogen atom, and one nitrogen atom. Seven electrons (green) bind to the nucleus, successively occupying available electron shells (rings). An atom is composed of two regions: Nitrogen is in group 5 of the periodic table with the electronic configuration 1s 2 2s 2 2p 3.therefore, the lone nitrogen atom contributes 5 x 1 = 5 valence electrons. Electrons are revolving around the central nucleus in the circular path. Aug 04, 2021 · atomic structure of nitrogen. Please visit the nitrogen element page for information specific to the chemical element of the periodic table.. Jul 26, 2021 · we can use these electrons to form the lewis structure for nitrous acid.

The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons... Seven electrons (white) occupy available electron shells (rings). The nucleus is composed of protons and neutrons.. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen.
I show you where nitrogen is on the periodic table and how to determine.. The nucleus consists of 7 protons (red) and 7 neutrons (orange). Laboratory chemical safety summary (lcss) datasheet.. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.

The nucleus is composed of protons and neutrons... The chemical symbol for nitrogen is n.. Nitrous acid comprises two oxygen atoms, one hydrogen atom, and one nitrogen atom.

Please visit the nitrogen element page for information specific to the chemical element of the periodic table... Since the number of protons is equal to the number of neutrons, the nitrogen atom is electrically neutral. Laboratory chemical safety summary (lcss) datasheet. Nitrogen is in group 5 of the periodic table with the electronic configuration 1s 2 2s 2 2p 3.therefore, the lone nitrogen atom contributes 5 x 1 = 5 valence electrons. The nitrogen \(\left( {\rm{n}} \right)\) is made of \(7\) protons, \(7\) electrons and \(7\) neutrons. Electrons are revolving around the central nucleus in the circular path.
Laboratory chemical safety summary (lcss) datasheet.. Since the number of protons is equal to the number of neutrons, the nitrogen atom is electrically neutral. Seven electrons (white) occupy available electron shells (rings). The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. I show you where nitrogen is on the periodic table and how to determine. The nitrogen \(\left( {\rm{n}} \right)\) is made of \(7\) protons, \(7\) electrons and \(7\) neutrons. Jul 26, 2021 · we can use these electrons to form the lewis structure for nitrous acid. Laboratory chemical safety summary (lcss) datasheet. Nitrous acid comprises two oxygen atoms, one hydrogen atom, and one nitrogen atom.. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.

The nucleus consists of 7 protons (red) and 7 neutrons (blue).. 7), the most common isotope of the element nitrogen.. Since the number of protons is equal to the number of neutrons, the nitrogen atom is electrically neutral.

Please visit the nitrogen element page for information specific to the chemical element of the periodic table.. .. I show you where nitrogen is on the periodic table and how to determine.

Electrons are revolving around the central nucleus in the circular path. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen.. Aug 04, 2021 · atomic structure of nitrogen.

Since the number of protons is equal to the number of neutrons, the nitrogen atom is electrically neutral. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons... Aug 04, 2021 · atomic structure of nitrogen.

Seven electrons (white) occupy available electron shells (rings). The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. The nitrogen \(\left( {\rm{n}} \right)\) is made of \(7\) protons, \(7\) electrons and \(7\) neutrons. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. Aug 04, 2021 · atomic structure of nitrogen... Electrons are revolving around the central nucleus in the circular path.

Please visit the nitrogen element page for information specific to the chemical element of the periodic table. Nitrogen is in group 5 of the periodic table with the electronic configuration 1s 2 2s 2 2p 3.therefore, the lone nitrogen atom contributes 5 x 1 = 5 valence electrons. The chemical symbol for nitrogen is n. The nucleus is composed of protons and neutrons.

An atom is composed of two regions: 7), the most common isotope of the element nitrogen.

Please visit the nitrogen element page for information specific to the chemical element of the periodic table. 7), the most common isotope of the element nitrogen. Aug 04, 2021 · atomic structure of nitrogen.. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen.

Electrons are revolving around the central nucleus in the circular path... Please visit the nitrogen element page for information specific to the chemical element of the periodic table. Aug 04, 2021 · atomic structure of nitrogen. Electrons are revolving around the central nucleus in the circular path.

Aug 04, 2021 · atomic structure of nitrogen.. Aug 04, 2021 · atomic structure of nitrogen... Nitrogen is in group 5 of the periodic table with the electronic configuration 1s 2 2s 2 2p 3.therefore, the lone nitrogen atom contributes 5 x 1 = 5 valence electrons.

The chemical symbol for nitrogen is n... The nucleus consists of 7 protons (red) and 7 neutrons (orange). The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The chemical symbol for nitrogen is n... Electrons are revolving around the central nucleus in the circular path.

Laboratory chemical safety summary (lcss) datasheet. 7), the most common isotope of the element nitrogen. An atom is composed of two regions: Jul 26, 2021 · we can use these electrons to form the lewis structure for nitrous acid. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. Please visit the nitrogen element page for information specific to the chemical element of the periodic table. I show you where nitrogen is on the periodic table and how to determine. The nucleus consists of 7 protons (red) and 7 neutrons (blue). The nucleus consists of 7 protons (red) and 7 neutrons (orange). The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen... Nitrous acid comprises two oxygen atoms, one hydrogen atom, and one nitrogen atom.

Aug 04, 2021 · atomic structure of nitrogen... Aug 04, 2021 · atomic structure of nitrogen. Since the number of protons is equal to the number of neutrons, the nitrogen atom is electrically neutral. The nucleus consists of 7 protons (red) and 7 neutrons (orange). Nitrogen is in group 5 of the periodic table with the electronic configuration 1s 2 2s 2 2p 3.therefore, the lone nitrogen atom contributes 5 x 1 = 5 valence electrons. The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. Electrons are revolving around the central nucleus in the circular path. 7), the most common isotope of the element nitrogen. An atom is composed of two regions: Seven electrons (green) bind to the nucleus, successively occupying available electron shells (rings).. Laboratory chemical safety summary (lcss) datasheet.

The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. Electrons are revolving around the central nucleus in the circular path. Since the number of protons is equal to the number of neutrons, the nitrogen atom is electrically neutral. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. Nitrogen is in group 5 of the periodic table with the electronic configuration 1s 2 2s 2 2p 3.therefore, the lone nitrogen atom contributes 5 x 1 = 5 valence electrons. I show you where nitrogen is on the periodic table and how to determine... Please visit the nitrogen element page for information specific to the chemical element of the periodic table.

The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus... The chemical symbol for nitrogen is n. Seven electrons (white) occupy available electron shells (rings). Seven electrons (green) bind to the nucleus, successively occupying available electron shells (rings). 7), the most common isotope of the element nitrogen.. Aug 04, 2021 · atomic structure of nitrogen.

The nucleus consists of 7 protons (red) and 7 neutrons (orange). Jul 26, 2021 · we can use these electrons to form the lewis structure for nitrous acid. An atom is composed of two regions: Aug 04, 2021 · atomic structure of nitrogen.. The nucleus consists of 7 protons (red) and 7 neutrons (blue).

I show you where nitrogen is on the periodic table and how to determine... Aug 04, 2021 · atomic structure of nitrogen. Since the number of protons is equal to the number of neutrons, the nitrogen atom is electrically neutral. The nucleus consists of 7 protons (red) and 7 neutrons (blue). An atom is composed of two regions: Seven electrons (green) bind to the nucleus, successively occupying available electron shells (rings). The nucleus is composed of protons and neutrons. 7), the most common isotope of the element nitrogen. Nov 21, 2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure... The nitrogen \(\left( {\rm{n}} \right)\) is made of \(7\) protons, \(7\) electrons and \(7\) neutrons.

Nitrous acid comprises two oxygen atoms, one hydrogen atom, and one nitrogen atom. The chemical symbol for nitrogen is n. Nitrous acid comprises two oxygen atoms, one hydrogen atom, and one nitrogen atom. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus... The nucleus consists of 7 protons (red) and 7 neutrons (blue).

The nucleus consists of 7 protons (red) and 7 neutrons (orange)... Nitrous acid comprises two oxygen atoms, one hydrogen atom, and one nitrogen atom. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. Electrons are revolving around the central nucleus in the circular path. The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. The chemical symbol for nitrogen is n. The nucleus consists of 7 protons (red) and 7 neutrons (orange). The nucleus is composed of protons and neutrons. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen.. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen.

Nov 21, 2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure.. . The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state.

The nucleus consists of 7 protons (red) and 7 neutrons (orange)... Seven electrons (green) bind to the nucleus, successively occupying available electron shells (rings). Laboratory chemical safety summary (lcss) datasheet. The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. 7), the most common isotope of the element nitrogen. Nitrous acid comprises two oxygen atoms, one hydrogen atom, and one nitrogen atom. 7), the most common isotope of the element nitrogen. The nucleus consists of 7 protons (red) and 7 neutrons (blue).. The nucleus consists of 7 protons (red) and 7 neutrons (orange).

The nucleus is composed of protons and neutrons... Seven electrons (green) bind to the nucleus, successively occupying available electron shells (rings). I show you where nitrogen is on the periodic table and how to determine. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. Laboratory chemical safety summary (lcss) datasheet.

Nov 21, 2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure.. Nitrous acid comprises two oxygen atoms, one hydrogen atom, and one nitrogen atom. I show you where nitrogen is on the periodic table and how to determine. Please visit the nitrogen element page for information specific to the chemical element of the periodic table. Nitrogen is in group 5 of the periodic table with the electronic configuration 1s 2 2s 2 2p 3.therefore, the lone nitrogen atom contributes 5 x 1 = 5 valence electrons. An atom is composed of two regions: Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. The nucleus is composed of protons and neutrons. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. The nucleus consists of 7 protons (red) and 7 neutrons (orange)... 7), the most common isotope of the element nitrogen.
The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons... The nucleus consists of 7 protons (red) and 7 neutrons (blue). 7), the most common isotope of the element nitrogen. An atom is composed of two regions: The nucleus consists of 7 protons (red) and 7 neutrons (orange). The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. The chemical symbol for nitrogen is n. Seven electrons (green) bind to the nucleus, successively occupying available electron shells (rings). The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.. Nitrogen is in group 5 of the periodic table with the electronic configuration 1s 2 2s 2 2p 3.therefore, the lone nitrogen atom contributes 5 x 1 = 5 valence electrons.

Nitrous acid comprises two oxygen atoms, one hydrogen atom, and one nitrogen atom. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. 7), the most common isotope of the element nitrogen. Seven electrons (white) occupy available electron shells (rings). Electrons are revolving around the central nucleus in the circular path.

Electrons are revolving around the central nucleus in the circular path. Since the number of protons is equal to the number of neutrons, the nitrogen atom is electrically neutral. The nucleus consists of 7 protons (red) and 7 neutrons (blue). Electrons are revolving around the central nucleus in the circular path. An atom is composed of two regions:
7), the most common isotope of the element nitrogen. Nitrogen is in group 5 of the periodic table with the electronic configuration 1s 2 2s 2 2p 3.therefore, the lone nitrogen atom contributes 5 x 1 = 5 valence electrons. Seven electrons (green) bind to the nucleus, successively occupying available electron shells (rings). 7), the most common isotope of the element nitrogen. Electrons are revolving around the central nucleus in the circular path... An atom is composed of two regions:

The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. .. I show you where nitrogen is on the periodic table and how to determine.

The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Since the number of protons is equal to the number of neutrons, the nitrogen atom is electrically neutral.

I show you where nitrogen is on the periodic table and how to determine.. Aug 04, 2021 · atomic structure of nitrogen. The nucleus consists of 7 protons (red) and 7 neutrons (blue). Seven electrons (white) occupy available electron shells (rings). Jul 26, 2021 · we can use these electrons to form the lewis structure for nitrous acid. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. Seven electrons (green) bind to the nucleus, successively occupying available electron shells (rings). The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Electrons are revolving around the central nucleus in the circular path.

An atom is composed of two regions:. 7), the most common isotope of the element nitrogen. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.

Nitrous acid comprises two oxygen atoms, one hydrogen atom, and one nitrogen atom. I show you where nitrogen is on the periodic table and how to determine. The nucleus consists of 7 protons (red) and 7 neutrons (blue). Seven electrons (white) occupy available electron shells (rings). Aug 04, 2021 · atomic structure of nitrogen. The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. An atom is composed of two regions: Nov 21, 2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state... The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus.

The nucleus is composed of protons and neutrons... The nucleus consists of 7 protons (red) and 7 neutrons (blue). The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. The nucleus consists of 7 protons (red) and 7 neutrons (orange). Since the number of protons is equal to the number of neutrons, the nitrogen atom is electrically neutral. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. The chemical symbol for nitrogen is n. Seven electrons (white) occupy available electron shells (rings).. Seven electrons (white) occupy available electron shells (rings).

Jul 26, 2021 · we can use these electrons to form the lewis structure for nitrous acid... Nitrogen is in group 5 of the periodic table with the electronic configuration 1s 2 2s 2 2p 3.therefore, the lone nitrogen atom contributes 5 x 1 = 5 valence electrons.. Aug 04, 2021 · atomic structure of nitrogen.
The nucleus consists of 7 protons (red) and 7 neutrons (orange).. The nucleus consists of 7 protons (red) and 7 neutrons (blue). The nucleus is composed of protons and neutrons. Nitrogen is in group 5 of the periodic table with the electronic configuration 1s 2 2s 2 2p 3.therefore, the lone nitrogen atom contributes 5 x 1 = 5 valence electrons. I show you where nitrogen is on the periodic table and how to determine. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. Jul 26, 2021 · we can use these electrons to form the lewis structure for nitrous acid. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen. Seven electrons (white) occupy available electron shells (rings). Nov 21, 2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. Nitrous acid comprises two oxygen atoms, one hydrogen atom, and one nitrogen atom... Nitrogen is in group 5 of the periodic table with the electronic configuration 1s 2 2s 2 2p 3.therefore, the lone nitrogen atom contributes 5 x 1 = 5 valence electrons.
Electrons are revolving around the central nucleus in the circular path. 7), the most common isotope of the element nitrogen. I show you where nitrogen is on the periodic table and how to determine. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. Elemental nitrogen consists of diatomic molecules so the element is frequently referred to as dinitrogen.. Aug 04, 2021 · atomic structure of nitrogen.

7), the most common isotope of the element nitrogen. Seven electrons (green) bind to the nucleus, successively occupying available electron shells (rings). 7), the most common isotope of the element nitrogen. The nucleus consists of 7 protons (red) and 7 neutrons (blue). Electrons are revolving around the central nucleus in the circular path. The nitrogen \(\left( {\rm{n}} \right)\) is made of \(7\) protons, \(7\) electrons and \(7\) neutrons. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. Please visit the nitrogen element page for information specific to the chemical element of the periodic table. The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. The nucleus is composed of protons and neutrons... Electrons are revolving around the central nucleus in the circular path.

The chemical symbol for nitrogen is n... Nitrogen is in group 5 of the periodic table with the electronic configuration 1s 2 2s 2 2p 3.therefore, the lone nitrogen atom contributes 5 x 1 = 5 valence electrons... Electrons are revolving around the central nucleus in the circular path.

I show you where nitrogen is on the periodic table and how to determine. Since the number of protons is equal to the number of neutrons, the nitrogen atom is electrically neutral. The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n.
Nitrous acid comprises two oxygen atoms, one hydrogen atom, and one nitrogen atom. The nitrogen atom has a valence shell population of 2s2 2 p3 so it has a 4s ground state. Nov 21, 2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. Since the number of protons is equal to the number of neutrons, the nitrogen atom is electrically neutral. Please visit the nitrogen element page for information specific to the chemical element of the periodic table. The nucleus consists of 7 protons (red) and 7 neutrons (orange). Seven electrons (white) occupy available electron shells (rings). 7), the most common isotope of the element nitrogen. Electrons are revolving around the central nucleus in the circular path. The atoms are found to consist of two isotopes, 14 n (99.635%) and 15 n. The nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus.. The nucleus is composed of protons and neutrons.
