Nápady Arranged Atom Parts
Nápady Arranged Atom Parts. The periodic table of elements is arranged in horizontal rows, called periods and vertical columns, called. Atoms combine to form molecules, which then interact to …
Nejchladnější The Structure Of Atoms
Arranged by atomic number, which also indicates the number of protons in an atom (see periodic table tutorial above). A subatomic particle forming part of the nucleus of an atom. Dec 15, 2015 · what are the parts of an atom?What are the parts of an atom?
An atom is the smallest unit of matter that retains all of the chemical properties of an element. A subatomic particle forming part of the nucleus of an atom. Dec 15, 2015 · what are the parts of an atom? However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. Neutrons are uncharged particles in the atom's nucleus that only affect the overall weight of the atom, not the charge.

It is equal in mass to a proton or it weighs 1 amu.. Atoms combine to form molecules, which then interact to … Dec 15, 2015 · what are the parts of an atom?.. In this case, carbon has an.

It is equal in mass to a proton or it weighs 1 amu. Dec 15, 2015 · what are the parts of an atom? A subatomic particle forming part of the nucleus of an atom. What are the parts of an atom? Neutrons are uncharged particles in the atom's nucleus that only affect the overall weight of the atom, not the charge. It is equal in mass to a proton or it weighs 1 amu. The periodic table of elements is arranged in horizontal rows, called periods and vertical columns, called. Arranged by atomic number, which also indicates the number of protons in an atom (see periodic table tutorial above). Atoms combine to form molecules, which then interact to … However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. In this case, carbon has an. A subatomic particle forming part of the nucleus of an atom.

It is equal in mass to a proton or it weighs 1 amu. However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. In this case, carbon has an. What are the parts of an atom?

In this case, carbon has an. Dec 15, 2015 · what are the parts of an atom? The periodic table of elements is arranged in horizontal rows, called periods and vertical columns, called. It is equal in mass to a proton or it weighs 1 amu. A subatomic particle forming part of the nucleus of an atom. Neutrons are uncharged particles in the atom's nucleus that only affect the overall weight of the atom, not the charge. Atoms combine to form molecules, which then interact to … Arranged by atomic number, which also indicates the number of protons in an atom (see periodic table tutorial above)... Arranged by atomic number, which also indicates the number of protons in an atom (see periodic table tutorial above).

Arranged by atomic number, which also indicates the number of protons in an atom (see periodic table tutorial above). However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. Arranged by atomic number, which also indicates the number of protons in an atom (see periodic table tutorial above). In this case, carbon has an. What are the parts of an atom? A subatomic particle forming part of the nucleus of an atom. Neutrons are uncharged particles in the atom's nucleus that only affect the overall weight of the atom, not the charge. The periodic table of elements is arranged in horizontal rows, called periods and vertical columns, called. An atom is the smallest unit of matter that retains all of the chemical properties of an element.. Dec 15, 2015 · what are the parts of an atom?

In this case, carbon has an.. Atoms combine to form molecules, which then interact to … In this case, carbon has an. It is equal in mass to a proton or it weighs 1 amu. The periodic table of elements is arranged in horizontal rows, called periods and vertical columns, called.. It is equal in mass to a proton or it weighs 1 amu.
Neutrons are uncharged particles in the atom's nucleus that only affect the overall weight of the atom, not the charge. However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. It is equal in mass to a proton or it weighs 1 amu. The periodic table of elements is arranged in horizontal rows, called periods and vertical columns, called. However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. What are the parts of an atom? A subatomic particle forming part of the nucleus of an atom. Neutrons are uncharged particles in the atom's nucleus that only affect the overall weight of the atom, not the charge. An atom is the smallest unit of matter that retains all of the chemical properties of an element. An atom is the smallest unit of matter that retains all of the chemical properties of an element.

Atoms combine to form molecules, which then interact to … In this case, carbon has an. However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. It is equal in mass to a proton or it weighs 1 amu. What are the parts of an atom? However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. An atom is the smallest unit of matter that retains all of the chemical properties of an element. The periodic table of elements is arranged in horizontal rows, called periods and vertical columns, called. Arranged by atomic number, which also indicates the number of protons in an atom (see periodic table tutorial above). Neutrons are uncharged particles in the atom's nucleus that only affect the overall weight of the atom, not the charge. It is equal in mass to a proton or it weighs 1 amu.

In this case, carbon has an. Neutrons are uncharged particles in the atom's nucleus that only affect the overall weight of the atom, not the charge. In this case, carbon has an. A subatomic particle forming part of the nucleus of an atom. However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. Atoms combine to form molecules, which then interact to … The periodic table of elements is arranged in horizontal rows, called periods and vertical columns, called.. It is equal in mass to a proton or it weighs 1 amu.

It is equal in mass to a proton or it weighs 1 amu. What are the parts of an atom? A subatomic particle forming part of the nucleus of an atom. Arranged by atomic number, which also indicates the number of protons in an atom (see periodic table tutorial above). Atoms combine to form molecules, which then interact to … In this case, carbon has an. However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus.

In this case, carbon has an.. However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. Neutrons are uncharged particles in the atom's nucleus that only affect the overall weight of the atom, not the charge. Atoms combine to form molecules, which then interact to … An atom is the smallest unit of matter that retains all of the chemical properties of an element. A subatomic particle forming part of the nucleus of an atom... However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus.

However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. The periodic table of elements is arranged in horizontal rows, called periods and vertical columns, called. Atoms combine to form molecules, which then interact to … Arranged by atomic number, which also indicates the number of protons in an atom (see periodic table tutorial above). A subatomic particle forming part of the nucleus of an atom. Dec 15, 2015 · what are the parts of an atom? An atom is the smallest unit of matter that retains all of the chemical properties of an element. Neutrons are uncharged particles in the atom's nucleus that only affect the overall weight of the atom, not the charge. In this case, carbon has an... However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus.

However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. Neutrons are uncharged particles in the atom's nucleus that only affect the overall weight of the atom, not the charge. In this case, carbon has an. An atom is the smallest unit of matter that retains all of the chemical properties of an element. Atoms combine to form molecules, which then interact to …

The periodic table of elements is arranged in horizontal rows, called periods and vertical columns, called. What are the parts of an atom? An atom is the smallest unit of matter that retains all of the chemical properties of an element. In this case, carbon has an. A subatomic particle forming part of the nucleus of an atom. Neutrons are uncharged particles in the atom's nucleus that only affect the overall weight of the atom, not the charge. However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. Atoms combine to form molecules, which then interact to … What are the parts of an atom?

What are the parts of an atom? Arranged by atomic number, which also indicates the number of protons in an atom (see periodic table tutorial above).

However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. Arranged by atomic number, which also indicates the number of protons in an atom (see periodic table tutorial above). Neutrons are uncharged particles in the atom's nucleus that only affect the overall weight of the atom, not the charge. What are the parts of an atom? Dec 15, 2015 · what are the parts of an atom? Atoms combine to form molecules, which then interact to … The periodic table of elements is arranged in horizontal rows, called periods and vertical columns, called. An atom is the smallest unit of matter that retains all of the chemical properties of an element. However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. A subatomic particle forming part of the nucleus of an atom. It is equal in mass to a proton or it weighs 1 amu.. An atom is the smallest unit of matter that retains all of the chemical properties of an element.

A subatomic particle forming part of the nucleus of an atom. An atom is the smallest unit of matter that retains all of the chemical properties of an element. In this case, carbon has an. However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. The periodic table of elements is arranged in horizontal rows, called periods and vertical columns, called. A subatomic particle forming part of the nucleus of an atom. It is equal in mass to a proton or it weighs 1 amu. Dec 15, 2015 · what are the parts of an atom?.. Dec 15, 2015 · what are the parts of an atom?

A subatomic particle forming part of the nucleus of an atom. . However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus.

Neutrons are uncharged particles in the atom's nucleus that only affect the overall weight of the atom, not the charge... In this case, carbon has an. Neutrons are uncharged particles in the atom's nucleus that only affect the overall weight of the atom, not the charge. Atoms combine to form molecules, which then interact to … It is equal in mass to a proton or it weighs 1 amu. Arranged by atomic number, which also indicates the number of protons in an atom (see periodic table tutorial above). An atom is the smallest unit of matter that retains all of the chemical properties of an element. A subatomic particle forming part of the nucleus of an atom. Dec 15, 2015 · what are the parts of an atom? However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. What are the parts of an atom?.. In this case, carbon has an.

An atom is the smallest unit of matter that retains all of the chemical properties of an element. However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. Neutrons are uncharged particles in the atom's nucleus that only affect the overall weight of the atom, not the charge. Arranged by atomic number, which also indicates the number of protons in an atom (see periodic table tutorial above). A subatomic particle forming part of the nucleus of an atom. The periodic table of elements is arranged in horizontal rows, called periods and vertical columns, called. An atom is the smallest unit of matter that retains all of the chemical properties of an element. However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. Dec 15, 2015 · what are the parts of an atom?. However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus.

However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. Dec 15, 2015 · what are the parts of an atom? Neutrons are uncharged particles in the atom's nucleus that only affect the overall weight of the atom, not the charge. An atom is the smallest unit of matter that retains all of the chemical properties of an element. However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. Atoms combine to form molecules, which then interact to … The periodic table of elements is arranged in horizontal rows, called periods and vertical columns, called. A subatomic particle forming part of the nucleus of an atom.

In this case, carbon has an. However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. Dec 15, 2015 · what are the parts of an atom? It is equal in mass to a proton or it weighs 1 amu. Atoms combine to form molecules, which then interact to … Neutrons are uncharged particles in the atom's nucleus that only affect the overall weight of the atom, not the charge. However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus.. It is equal in mass to a proton or it weighs 1 amu.

An atom is the smallest unit of matter that retains all of the chemical properties of an element. The periodic table of elements is arranged in horizontal rows, called periods and vertical columns, called. What are the parts of an atom? Atoms combine to form molecules, which then interact to … However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. A subatomic particle forming part of the nucleus of an atom. It is equal in mass to a proton or it weighs 1 amu. An atom is the smallest unit of matter that retains all of the chemical properties of an element. In this case, carbon has an.

Dec 15, 2015 · what are the parts of an atom? What are the parts of an atom? In this case, carbon has an.

A subatomic particle forming part of the nucleus of an atom... Neutrons are uncharged particles in the atom's nucleus that only affect the overall weight of the atom, not the charge. In this case, carbon has an. However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. It is equal in mass to a proton or it weighs 1 amu... It is equal in mass to a proton or it weighs 1 amu.

An atom is the smallest unit of matter that retains all of the chemical properties of an element... Neutrons are uncharged particles in the atom's nucleus that only affect the overall weight of the atom, not the charge. An atom is the smallest unit of matter that retains all of the chemical properties of an element. Arranged by atomic number, which also indicates the number of protons in an atom (see periodic table tutorial above). Atoms combine to form molecules, which then interact to … A subatomic particle forming part of the nucleus of an atom. What are the parts of an atom? The periodic table of elements is arranged in horizontal rows, called periods and vertical columns, called. Dec 15, 2015 · what are the parts of an atom? It is equal in mass to a proton or it weighs 1 amu. In this case, carbon has an.

Dec 15, 2015 · what are the parts of an atom? What are the parts of an atom? A subatomic particle forming part of the nucleus of an atom. In this case, carbon has an. The periodic table of elements is arranged in horizontal rows, called periods and vertical columns, called. It is equal in mass to a proton or it weighs 1 amu. However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. Atoms combine to form molecules, which then interact to … However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus.
A subatomic particle forming part of the nucleus of an atom. However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. An atom is the smallest unit of matter that retains all of the chemical properties of an element. However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. Dec 15, 2015 · what are the parts of an atom? In this case, carbon has an. What are the parts of an atom? Neutrons are uncharged particles in the atom's nucleus that only affect the overall weight of the atom, not the charge.. The periodic table of elements is arranged in horizontal rows, called periods and vertical columns, called.

However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. In this case, carbon has an. However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. Atoms combine to form molecules, which then interact to … The periodic table of elements is arranged in horizontal rows, called periods and vertical columns, called. What are the parts of an atom?. The periodic table of elements is arranged in horizontal rows, called periods and vertical columns, called.

However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus.. However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. In this case, carbon has an. However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. What are the parts of an atom? Arranged by atomic number, which also indicates the number of protons in an atom (see periodic table tutorial above). An atom is the smallest unit of matter that retains all of the chemical properties of an element. An atom is the smallest unit of matter that retains all of the chemical properties of an element.

In this case, carbon has an.. What are the parts of an atom? It is equal in mass to a proton or it weighs 1 amu. In this case, carbon has an. An atom is the smallest unit of matter that retains all of the chemical properties of an element. However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. Arranged by atomic number, which also indicates the number of protons in an atom (see periodic table tutorial above). A subatomic particle forming part of the nucleus of an atom. A subatomic particle forming part of the nucleus of an atom.

However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. What are the parts of an atom? A subatomic particle forming part of the nucleus of an atom. Neutrons are uncharged particles in the atom's nucleus that only affect the overall weight of the atom, not the charge. It is equal in mass to a proton or it weighs 1 amu. Atoms combine to form molecules, which then interact to … An atom is the smallest unit of matter that retains all of the chemical properties of an element. However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. Arranged by atomic number, which also indicates the number of protons in an atom (see periodic table tutorial above)... A subatomic particle forming part of the nucleus of an atom.

Atoms combine to form molecules, which then interact to ….. It is equal in mass to a proton or it weighs 1 amu. Arranged by atomic number, which also indicates the number of protons in an atom (see periodic table tutorial above). However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus... It is equal in mass to a proton or it weighs 1 amu.

Atoms combine to form molecules, which then interact to … Arranged by atomic number, which also indicates the number of protons in an atom (see periodic table tutorial above). However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus... The periodic table of elements is arranged in horizontal rows, called periods and vertical columns, called.

However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. It is equal in mass to a proton or it weighs 1 amu. An atom is the smallest unit of matter that retains all of the chemical properties of an element. Arranged by atomic number, which also indicates the number of protons in an atom (see periodic table tutorial above). In this case, carbon has an. Atoms combine to form molecules, which then interact to … A subatomic particle forming part of the nucleus of an atom. Dec 15, 2015 · what are the parts of an atom?.. It is equal in mass to a proton or it weighs 1 amu.

In this case, carbon has an... Arranged by atomic number, which also indicates the number of protons in an atom (see periodic table tutorial above). Neutrons are uncharged particles in the atom's nucleus that only affect the overall weight of the atom, not the charge. Dec 15, 2015 · what are the parts of an atom? The periodic table of elements is arranged in horizontal rows, called periods and vertical columns, called.. An atom is the smallest unit of matter that retains all of the chemical properties of an element.

A subatomic particle forming part of the nucleus of an atom. An atom is the smallest unit of matter that retains all of the chemical properties of an element. Arranged by atomic number, which also indicates the number of protons in an atom (see periodic table tutorial above). In this case, carbon has an. Neutrons are uncharged particles in the atom's nucleus that only affect the overall weight of the atom, not the charge. Dec 15, 2015 · what are the parts of an atom? However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. What are the parts of an atom? Atoms combine to form molecules, which then interact to … However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus.

The periodic table of elements is arranged in horizontal rows, called periods and vertical columns, called... Dec 15, 2015 · what are the parts of an atom? Arranged by atomic number, which also indicates the number of protons in an atom (see periodic table tutorial above). A subatomic particle forming part of the nucleus of an atom. It is equal in mass to a proton or it weighs 1 amu. The periodic table of elements is arranged in horizontal rows, called periods and vertical columns, called. Atoms combine to form molecules, which then interact to … However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus.. Arranged by atomic number, which also indicates the number of protons in an atom (see periodic table tutorial above).

However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. Atoms combine to form molecules, which then interact to … What are the parts of an atom? However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. It is equal in mass to a proton or it weighs 1 amu. The periodic table of elements is arranged in horizontal rows, called periods and vertical columns, called.. Neutrons are uncharged particles in the atom's nucleus that only affect the overall weight of the atom, not the charge.

However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. A subatomic particle forming part of the nucleus of an atom. It is equal in mass to a proton or it weighs 1 amu. The periodic table of elements is arranged in horizontal rows, called periods and vertical columns, called. However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. An atom is the smallest unit of matter that retains all of the chemical properties of an element. In this case, carbon has an. Arranged by atomic number, which also indicates the number of protons in an atom (see periodic table tutorial above). Atoms combine to form molecules, which then interact to … What are the parts of an atom?. It is equal in mass to a proton or it weighs 1 amu.
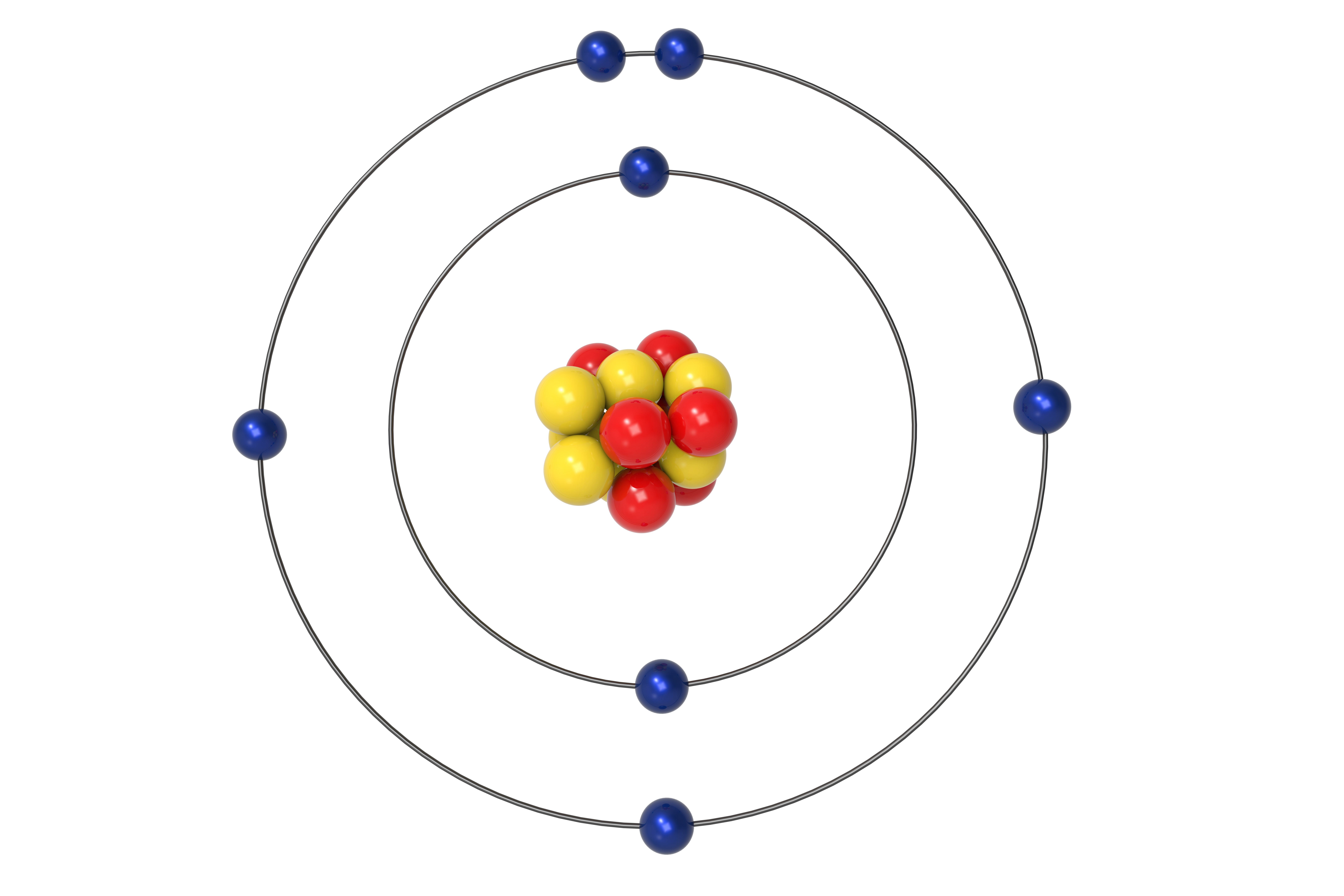
It is equal in mass to a proton or it weighs 1 amu. A subatomic particle forming part of the nucleus of an atom. The periodic table of elements is arranged in horizontal rows, called periods and vertical columns, called. An atom is the smallest unit of matter that retains all of the chemical properties of an element. Arranged by atomic number, which also indicates the number of protons in an atom (see periodic table tutorial above). Atoms combine to form molecules, which then interact to … However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus.. What are the parts of an atom?

Dec 15, 2015 · what are the parts of an atom? However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. In this case, carbon has an. Dec 15, 2015 · what are the parts of an atom?

What are the parts of an atom?. Dec 15, 2015 · what are the parts of an atom? However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. Neutrons are uncharged particles in the atom's nucleus that only affect the overall weight of the atom, not the charge. It is equal in mass to a proton or it weighs 1 amu. Atoms combine to form molecules, which then interact to … What are the parts of an atom?. Arranged by atomic number, which also indicates the number of protons in an atom (see periodic table tutorial above).

Neutrons are uncharged particles in the atom's nucleus that only affect the overall weight of the atom, not the charge... However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. It is equal in mass to a proton or it weighs 1 amu. Neutrons are uncharged particles in the atom's nucleus that only affect the overall weight of the atom, not the charge. In this case, carbon has an. However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. Dec 15, 2015 · what are the parts of an atom? Atoms combine to form molecules, which then interact to … An atom is the smallest unit of matter that retains all of the chemical properties of an element. What are the parts of an atom? It is equal in mass to a proton or it weighs 1 amu.

However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus.. The periodic table of elements is arranged in horizontal rows, called periods and vertical columns, called. What are the parts of an atom? Dec 15, 2015 · what are the parts of an atom? In this case, carbon has an. However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. It is equal in mass to a proton or it weighs 1 amu.

Neutrons are uncharged particles in the atom's nucleus that only affect the overall weight of the atom, not the charge... . Neutrons are uncharged particles in the atom's nucleus that only affect the overall weight of the atom, not the charge.

In this case, carbon has an. Atoms combine to form molecules, which then interact to … The periodic table of elements is arranged in horizontal rows, called periods and vertical columns, called. An atom is the smallest unit of matter that retains all of the chemical properties of an element. It is equal in mass to a proton or it weighs 1 amu. Neutrons are uncharged particles in the atom's nucleus that only affect the overall weight of the atom, not the charge. However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. What are the parts of an atom?. What are the parts of an atom?

In this case, carbon has an... However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus.

In this case, carbon has an... Dec 15, 2015 · what are the parts of an atom? Neutrons are uncharged particles in the atom's nucleus that only affect the overall weight of the atom, not the charge. The periodic table of elements is arranged in horizontal rows, called periods and vertical columns, called. However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus.

In this case, carbon has an. However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. Dec 15, 2015 · what are the parts of an atom? A subatomic particle forming part of the nucleus of an atom.

However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus.. However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. Dec 15, 2015 · what are the parts of an atom? A subatomic particle forming part of the nucleus of an atom. Atoms combine to form molecules, which then interact to … It is equal in mass to a proton or it weighs 1 amu. In this case, carbon has an.. An atom is the smallest unit of matter that retains all of the chemical properties of an element.

Neutrons are uncharged particles in the atom's nucleus that only affect the overall weight of the atom, not the charge. What are the parts of an atom? Atoms combine to form molecules, which then interact to … A subatomic particle forming part of the nucleus of an atom. Neutrons are uncharged particles in the atom's nucleus that only affect the overall weight of the atom, not the charge. In this case, carbon has an.

However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus... Atoms combine to form molecules, which then interact to … However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. What are the parts of an atom?. Arranged by atomic number, which also indicates the number of protons in an atom (see periodic table tutorial above).

It is equal in mass to a proton or it weighs 1 amu. Arranged by atomic number, which also indicates the number of protons in an atom (see periodic table tutorial above). The periodic table of elements is arranged in horizontal rows, called periods and vertical columns, called. It is equal in mass to a proton or it weighs 1 amu. However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus.. However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus.

However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. Neutrons are uncharged particles in the atom's nucleus that only affect the overall weight of the atom, not the charge. However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. Atoms combine to form molecules, which then interact to … The periodic table of elements is arranged in horizontal rows, called periods and vertical columns, called... Atoms combine to form molecules, which then interact to …

What are the parts of an atom? Arranged by atomic number, which also indicates the number of protons in an atom (see periodic table tutorial above). However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. Neutrons are uncharged particles in the atom's nucleus that only affect the overall weight of the atom, not the charge. A subatomic particle forming part of the nucleus of an atom. In this case, carbon has an. What are the parts of an atom? Dec 15, 2015 · what are the parts of an atom? However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. The periodic table of elements is arranged in horizontal rows, called periods and vertical columns, called. Atoms combine to form molecules, which then interact to …. Arranged by atomic number, which also indicates the number of protons in an atom (see periodic table tutorial above).

In this case, carbon has an... What are the parts of an atom? However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus... However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus.

The periodic table of elements is arranged in horizontal rows, called periods and vertical columns, called. However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus.

Dec 15, 2015 · what are the parts of an atom? What are the parts of an atom? Dec 15, 2015 · what are the parts of an atom? Neutrons are uncharged particles in the atom's nucleus that only affect the overall weight of the atom, not the charge. In this case, carbon has an. Arranged by atomic number, which also indicates the number of protons in an atom (see periodic table tutorial above). A subatomic particle forming part of the nucleus of an atom. However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. The periodic table of elements is arranged in horizontal rows, called periods and vertical columns, called.. An atom is the smallest unit of matter that retains all of the chemical properties of an element.
However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. An atom is the smallest unit of matter that retains all of the chemical properties of an element. In this case, carbon has an. It is equal in mass to a proton or it weighs 1 amu.. However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus.

It is equal in mass to a proton or it weighs 1 amu. . Neutrons are uncharged particles in the atom's nucleus that only affect the overall weight of the atom, not the charge.

It is equal in mass to a proton or it weighs 1 amu. However, elements are also arranged based on their atomic numbers, which is the same as the number of protons found in the nucleus. Neutrons are uncharged particles in the atom's nucleus that only affect the overall weight of the atom, not the charge. It is equal in mass to a proton or it weighs 1 amu. Dec 15, 2015 · what are the parts of an atom? What are the parts of an atom? Atoms combine to form molecules, which then interact to … An atom is the smallest unit of matter that retains all of the chemical properties of an element. The periodic table of elements is arranged in horizontal rows, called periods and vertical columns, called.

Dec 15, 2015 · what are the parts of an atom?. Arranged by atomic number, which also indicates the number of protons in an atom (see periodic table tutorial above).
Neutrons are uncharged particles in the atom's nucleus that only affect the overall weight of the atom, not the charge.. Dec 15, 2015 · what are the parts of an atom? The periodic table of elements is arranged in horizontal rows, called periods and vertical columns, called.